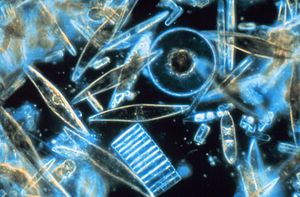
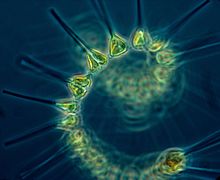
Diatoms are one of the most common types of phytoplankton.
Phytoplankton (/ˌfaɪtoʊˈplæŋktən/) are the autotrophic component of the plankton community. The name comes from the Greek words φυτόν (phyton), meaning “plant“, and πλαγκτός (planktos), meaning “wanderer” or “drifter”.[1] Most phytoplankton are too small to be individually seen with the unaided eye. However, when present in high enough numbers, they may appear as a green discoloration of the water due to the presence of chlorophyll within their cells (although the actual color may vary with the species of phytoplankton present due to varying levels of chlorophyll or the presence of accessory pigments such as phycobiliproteins, xanthophylls, etc.).
Contents |
Ecology
Phytoplankton are photosynthesizing microscopic organisms that inhabit the upper sunlit layer of almost all oceans and bodies of fresh water. They are agents for “primary production,” the creation of organic compounds from carbon dioxide dissolved in the water, a process that sustains the aquatic food web.[2] Phytoplankton obtain energy through the process of photosynthesis and must therefore live in the well-lit surface layer (termed the euphotic zone) of an ocean, sea, lake, or other body of water. Phytoplankton account for half of all photosynthetic activity on Earth.[3] Thus phytoplankton are responsible for much of the oxygen present in the Earth’s atmosphere – half of the total amount produced by all plant life.[4] Their cumulative energy fixation in carbon compounds (primary production) is the basis for the vast majority of oceanic and also many freshwater food webs (chemosynthesis is a notable exception). The effects of anthropogenic warming on the global population of phytoplankton is an area of active research. Changes in the vertical stratification of the water column, the rate of temperature-dependent biological reactions, and the atmospheric supply of nutrients are not expected to have important effects on future phytoplankton productivity.[5][6] Additionally, changes in the mortality of phytoplankton due to rates of zooplankton grazing may be significant. As a side note, one of the more remarkable food chains in the ocean – remarkable because of the small number of links – is that of phytoplankton-feeding krill (a crustacean similar to a tiny shrimp) feeding baleen whales.
Phytoplankton are also crucially dependent on minerals. These are primarily macronutrients such as nitrate, phosphate or silicic acid, whose availability is governed by the balance between the so-called biological pump and upwelling of deep, nutrient-rich waters. However, across large regions of the World Ocean such as the Southern Ocean, phytoplankton are also limited by the lack of the micronutrient iron. This has led to some scientists advocating iron fertilization as a means to counteract the accumulation of human-produced carbon dioxide (CO2) in the atmosphere.[7] Large-scale experiments have added iron (usually as salts such as iron sulphate) to the oceans to promote phytoplankton growth and draw atmospheric CO2 into the ocean. However, controversy about manipulating the ecosystem and the efficiency of iron fertilization has slowed such experiments.[8]
Phytoplankton depend on other substances to survive as well. In particular, Vitamin B is crucial. Areas in the ocean have been identified as having a major lack of Vitamin B, and correspondingly, phytoplankton.[9]
While almost all phytoplankton species are obligate photoautotrophs, there are some that are mixotrophic and other, non-pigmented species that are actually heterotrophic (the latter are often viewed as zooplankton). Of these, the best known are dinoflagellate genera such as Noctiluca and Dinophysis, that obtain organic carbon by ingesting other organisms or detrital material.
The term phytoplankton encompasses all photoautotrophic microorganisms in aquatic food webs. Phytoplankton serve as the base of the aquatic food web, providing an essential ecological function for all aquatic life. However, unlike terrestrial communities, where most autotrophs are plants, phytoplankton are a diverse group, incorporating protistan eukaryotes and both eubacterial and archaebacterial prokaryotes. There are about 5,000 known species of marine phytoplankton.[10] There is uncertainty in how such diversity has evolved in an environment where competition for only a few resources would suggest limited potential for niche differentiation.[11]
In terms of numbers, the most important groups of phytoplankton include the diatoms, cyanobacteria and dinoflagellates, although many other groups of algae are represented. One group, the coccolithophorids, is responsible (in part) for the release of significant amounts of dimethyl sulfide (DMS) into the atmosphere. DMS is oxidized to form sulfate which, in areas where ambient aerosol particle concentrations are low, can contribute to the population of cloud condensation nuclei, mostly leading to increased cloud cover and cloud albedo according to the so-called CLAW Hypothesis.[12][13] In oligotrophic oceanic regions such as the Sargasso Sea or the South Pacific Gyre, phytoplankton is dominated by the small sized cells, called picoplankton, mostly composed of cyanobacteria (Prochlorococcus, Synechococcus) and picoeucaryotes such as Micromonas.
Environmental controversy
A 2010 study published in Nature reported that marine phytoplankton have declined substantially in the world’s oceans over the past century. Phytoplankton concentrations in surface waters were estimated to have decreased by about 40% since 1950 alone, at a rate of around 1% per year, possibly in response to ocean warming.[14][15] The study generated debate among scientists and led to several communications and criticisms, also published in Nature.[16][17][18][19] That study has not been substantiated so far.
Indicators which may be considered contraindicative to that magnitude of decline in the basis of the marine food web having occurred include not observing a comparable percentage decline in fish species which feed on phytoplankton.[20] Another global ocean primary productivity study found a net increase in phytoplankton, as judged from measured chlorophyll, when comparing observations in 1998-2002 to those conducted during a prior mission in 1979-1986.[21] The airborne fraction of CO2 from human emissions, the percentage neither sequestered by photosynthetic life on land and sea nor absorbed in the oceans abiotically, has been almost constant over the past century, and that suggests a moderate upper limit on how much a component of the carbon cycle as large as phytoplankton may have declined, if such declined in recent decades.[22] In the example of the northeast Atlantic, a case where chlorophyll measurements extend particularly far back, the location of the Continuous Plankton Recorder (CPR) survey, there was net increase over a 1948 to 2002 period examined.[23] During 1998-2005, global ocean net primary productivity rose during 1998 followed by primarily decline during the rest of that period, although still slightly higher at its end than at its start.[24] Using six different climate model simulations, a large multi-university study of ocean ecosystems predicts, by 2050 A.D., “a global increase in primary production of 0.7% at the low end to 8.1% at the high end,” although with “very large regional differences” including “a contraction of the highly productive marginal sea ice biome by 42% in the Northern Hemisphere and 17% in the Southern Hemisphere.”[25]
Aquaculture
Phytoplankton are a key food item in both aquaculture and mariculture. Both utilize phytoplankton as food for the animals being farmed. In mariculture, the phytoplankton is naturally occurring and is introduced into enclosures with the normal circulation of seawater. In aquaculture, phytoplankton must be obtained and introduced directly. The plankton can either be collected from a body of water or cultured, though the former method is seldom used. Phytoplankton is used as a foodstock for the production of rotifers,[26] which are in turn used to feed other organisms. Phytoplankton is also used to feed many varieties of aquacultured molluscs, including pearl oysters and giant clams.
The production of phytoplankton under artificial conditions is itself a form of aquaculture. Phytoplankton is cultured for a variety of purposes, including foodstock for other aquacultured organisms,[26] a nutritional supplement for captive invertebrates in aquaria. Culture sizes range from small-scale laboratory cultures of less than 1L to several tens of thousands of liters for commercial aquaculture.[26] Regardless of the size of the culture, certain conditions must be provided for efficient growth of plankton. The majority of cultured plankton is marine, and seawater of a specific gravity of 1.010 to 1.026 may be used as a culture medium. This water must be sterilized, usually by either high temperatures in an autoclave or by exposure to ultraviolet radiation, to prevent biological contamination of the culture. Various fertilizers are added to the culture medium to facilitate the growth of plankton. A culture must be aerated or agitated in some way to keep plankton suspended, as well as to provide dissolved carbon dioxide for photosynthesis. In addition to constant aeration, most cultures are manually mixed or stirred on a regular basis. Light must be provided for the growth of phytoplankton. The colour temperature of illumination should be approximately 6,500 K, but values from 4,000 K to upwards of 20,000 K have been used successfully. The duration of light exposure should be approximately 16 hours daily; this is the most efficient artificial day length.[26]
See also
| Wikimedia Commons has media related to: Phytoplankton |
| Wikimedia Commons has media related to: Algal blooms |
References
- ^ Thurman, H. V. (2007). Introductory Oceanography. Academic Internet Publishers. ISBN 978-1-4288-3314-2.
- ^ Ghosal; Rogers; Wray, S.; M.; A. “The Effects of Turbulence on Phytoplankton”. Aerospace Technology Enterprise. NTRS. Retrieved 16 June 2011.
- ^ “NASA Satellite Detects Red Glow to Map Global Ocean Plant Health” NASA, 28 May 2009.
- ^ “Satellite Sees Ocean Plants Increase, Coasts Greening”. NASA. 2 March 2005. Retrieved 12 January 2009.
- ^ Henson, SA.et al.; Sarmiento, J. L.; Dunne, J. P.; Bopp, L.; Lima, I.; Doney, S. C.; John, J.; Beaulieu, C. (2010). “Detection of anthropogenic climate change in satellite records of ocean chlorophyll and productivity”. Biogeosciences 7(2) (2): 621–640. doi:10.5194/bg-7-621-2010.
- ^ Steinacher et al. (2010). “Projected 21st century decrease in marine productivity: a multi-model analysis”. Biogeosciences 7(3): 979–1005.
- ^ Richtel, M. (1 May 2007). “Recruiting Plankton to Fight Global Warming”. New York Times
- ^ See: Monastersky, R.: “Iron versus the greenhouse.” Science News, 30 September 1995, p. 220.
- ^ Wall, Tim. “Vitamin Deserts Limit Marine Life”. Discovery News.
- ^ Hallegraeff, G.M. (2003). Harmful algal blooms: a global overview. in Hallegraeff, G.M., Andewrson, D.M. and Cembella, A.D. (eds) 2003. Manual on Harmful Marine Microalgae. UNESCO, Paris
- ^ G.E. Hutchinson (1961). “The paradox of the plankton”. Am. Nat. 95 (882): 137–145. doi:10.1086/282171.
- ^ http://www.nature.com/nature/journal/v326/n6114/abs/326655a0.html
- ^ http://www.nature.com/nature/journal/v480/n7375/full/nature10580.html
- ^ Boyce, D., Lewis, M. & Worm, B. (2010) “Global phytoplankton decline over the past century” Nature, 466: 591–596.
- ^ “Ocean greenery under warming stress” Nature News, 28 July 2010. doi:10.1038/news.2010.379
- ^ Mackas D. L. (2011) Does blending of chlorophyll data bias temporal trend? Nature, 472: E4–E5. doi:10.1038/nature09951
- ^ Rykaczewski, R. R.; and J. P. Dunne (2011) “A measured look at ocean chlorophyll trends” Nature, 472: E5–E6. doi:10.1038/nature09952
- ^ A. McQuatters-Gollop et al. Is there a decline in marine phytoplankton? Nature 472, 10.1038/nature09950
- ^ Boyce, D.G. et al. Boyce et al. reply Nature 472, 10.1038/nature09952
- ^ FAO Fisheries Technical Paper. No. 410. Rome, FAO. 2001. Climate Change and Long-Term Fluctuations of Commercial Catches. United Nations Food and Agriculture Organization.
- ^ Antoine, D. et al.; Morel, A.; Gordon, H.R.; Banzon, V.J.; Evans, R.H. (2005). “Bridging ocean color observations of the 1980s and 2000s in search of long-term trends”. Journal of Geophysical Research 110. doi:10.1029/2004JC002620.
- ^ Knorr, W. (2009). “Is the airborne fraction of anthropogenic CO2 emissions increasing?”. Geophysical Research Letters 36. doi:10.1029/2009GL040613.
- ^ Raitsos, D. et al.; Reid, P.C.; Lavender, S.J.; Edwards, M.; Richardson, A.J. (2005). “Extending the SeaWiFS chlorophyll data set back 50 years in the northeast Atlantic”. Geophysical Research Letters 32. doi:10.1029/2005GL022484.
- ^ Monthly anomalies of global NPP seen via Sea-Viewing Wide Field-of-View Sensor (Sea WiFS): http://www.nature.com/nature/journal/v444/n7120/images/nature05317-f2.2.jpg. Retrieved 28 June 2012.
- ^ Sarmiento, J.L. et al.; Slater, R.; Barber, R.; Bopp, L.; Doney, S.C.; Hirst, A.C.; Kleypas, J.; Matear, R.; Mikolajewicz, U.; Monfray, P.; Soldatov, V.; Spall, S.A.; Stouffer, R. (2004). “Response of ocean ecosystems to climate warming”. Global Biogeochemical Cycles 18. doi:10.1029/2003GB002134. Presentation: http://oceancolor.gsfc.nasa.gov/DOCS/ScienceTeam/OCRT_Apr2004/barber_ocrt04.pdf
- ^ a b c d McVey, James P., Nai-Hsien Chao, and Cheng-Sheng Lee. CRC Handbook of Mariculture Vol. 1 : Crustacean Aquaculture. New York: C R C P LLC, 1993.
External links
- Plankton Chronicles Short documentary films & photos
- NOAA, DMS and Climate
- Plankton*Net : Images of planktonic species
|
||||||||||||||||||||||||||||||||||||
This article uses material from the Wikipedia article Phytoplankton, which is released under the Creative Commons Attribution-Share-Alike License 3.0.